Introduction and summary
As the United States and the rest of the world rush to develop an effective vaccine and treatments for COVID-19, it is critical that these therapies work for everyone. Unfortunately, clinical trials and research have historically prioritized white, cisgender men. Women, along with transgender men and nonbinary and gender-nonconforming people, have been excluded and underrepresented—with consequences for their health.1
No measures to treat and prevent the coronavirus will be truly successful if they are not effective for large swaths of the population; a failure to provide a vaccine and treatment that works for women is a failure to protect the public’s health more broadly. Therefore, to better understand and address the different ways in which COVID-19 and other health conditions manifest—including but not limited to common conditions that disproportionately affect women, such as lupus and fibroids—it is critical for research and data to include women—especially women of color and pregnant women—as well as transgender men and nonbinary and gender-nonconforming people.
Hundreds of trials are underway to develop treatments and vaccines to address the coronavirus pandemic.2 An influx of funding and partnerships may lead to a faster-than-ever vaccine development; an optimistic timeline puts an available vaccine in circulation in 12 to 18 months.3 However, the scramble to quickly develop treatments and vaccines also raises concerns that care will not be taken to recruit groups of diverse participants or use rigorous informed consent standards.4 The pandemic presents an immediate need—and opportunity—to address the exclusion of women from research and clinical trials. But efforts to include women must not be limited to the coronavirus—rather, they must go beyond it to ensure that women, especially women of color, are represented across medical and scientific research in the long term. In order to protect and improve women’s health, from pandemics to chronic conditions, it is necessary to build a research agenda that centers women.
At least two steps are needed to accomplish this and to develop adequate treatments. First, researchers and drug developers must collect disaggregated data across sex, gender, and other intersecting demographics. Second, researchers must ensure that women, especially women of color, are included in clinical trials for the development of treatments and vaccines. This must also involve ensuring pregnant and lactating people are represented when it is safe and ethical.
Before a disease or health condition can be treated or prevented, robust, disaggregated data are necessary to understand the disease and how it affects people throughout the population, including who is most affected or at risk and what drives that risk. In addition to sex-disaggregated data, it is necessary to understand the intersection of sex and gender with other demographic factors such as race, age, and pregnancy, as well as social factors such as housing and employment, that may affect people’s risk and response to disease. Furthermore, clinical trials are central to building an understanding of diseases and health conditions and to preventing or treating those conditions. However, women and people of color have long been excluded from clinical research, resulting in an incomplete picture of which therapies are most effective and safe for women, particularly women of color.
The clinical research community has also failed to adequately fund and research conditions that are experienced disproportionately by women and to develop treatments that take into consideration women’s physiological needs and disease presentations. Furthermore, research gaps remain for conditions that disproportionately affect women of color, particularly Black women. There are also limited data regarding how certain conditions, therapies, and dietary supplements affect pregnant and lactating people. This lack of research has in turn had a remarkable impact on therapies’ development, to the detriment of women’s health and safety. Yet inclusion in research alone is not enough: Researchers must also disaggregate clinical trial data by sex, gender, race, and other factors in order to accurately assess the effectiveness and potential harms of treatments for women of color and others who do not fit the mold of a cisgender, white man.
In this report, the authors detail the historical and ongoing exclusion of women from clinical trials and medical research and the detrimental effects such exclusion has had on women’s health, particularly for women of color as well as pregnant and lactating people. The report then offers policy recommendations to ensure that women across intersecting identities are included in the development of research and treatment for COVID-19 and across every stage of research generally going forward. The report’s recommendations include:
- Disaggregating data across multiple factors, such as sex, gender, and race, for prevalence and research findings
- Requiring clinical researchers to assess therapies’ impacts on women and pregnant people
- Encouraging researchers to engage in targeted outreach to guarantee adequate representation
- Pressing lawmakers, agencies, and the research community to build trust in communities where trust has been broken
- Ensuring Congress and federal agencies exercise their oversight and enforcement authority to guarantee equitable research
Overall, to safeguard women’s health, and the public’s health as a whole, it is critical to build a research agenda that centers women through representation in clinical trials and to disaggregate data to understand the different effects of conditions and their treatments on women. Implementing the recommendations in this report can help to ensure equitable, effective treatments for the current pandemic and beyond. In implementing these recommendations, policymakers and other stakeholders must atone for the history of coercion and exclusion.
Available data on the coronavirus are incomplete
Currently, the United States is not collecting complete data about COVID-19 infection and mortality rates, and the data that are available are not consistently disaggregated by sex and gender.5 Available data from other countries and individual states show that women and men have comparable infection rates but that men have greater mortality rates.6 However, the country’s inadequate testing capacity, as well as gaps in data reporting, make it difficult to develop an accurate picture of the virus’s impacts and prevalence.7
Furthermore, the disease is novel, and scientists’ and medical professionals’ understanding regarding its health impact, including the effects among different populations, continues to evolve. For instance, it was largely understood initially that young people, particularly children, without preexisting medical conditions were experiencing less severe adverse health outcomes as a result of the disease. But as the disease spread, more young people became hospitalized.8 Recently, medical experts discovered that a number of children who have had COVID-19 or been exposed to the virus have developed a condition called multisystem inflammatory syndrome in children, during which the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal organs can become inflamed, resulting in serious complications or even death.9 Even more, there are reports of young and middle-aged COVID-19 patients suffering strokes; the COVID-19 stroke patient is 15 years younger than the average stroke victim.10
This evolution in understanding demonstrates there are still a number of unknowns regarding how the disease affects people generally and differently. Even with limited information nationwide, disturbing data from states and localities have shown significant racial disparities in infection and death rates, particularly for Black; American Indian or Alaska Native; and Hispanic or Latinx people.11 These trends indicate that women of color may be particularly at risk of contracting the virus. The problem is exacerbated by the fact that these same communities lack access to necessary care and supports as a result of long-standing systemic racism and sexism in economics, health care, and housing, among other factors.12 The Centers for Disease Control and Prevention (CDC) recently issued guidelines to require laboratory and nonlaboratory systems to collect and submit data on race, ethnicity, age, and sex to state and local officials, but it is not yet clear whether this policy is being effectively implemented.13
Currently, there are many unknowns about the interaction between the coronavirus and pregnancy, but research on past infectious diseases has shown that pregnant people are often at greater risk of respiratory complications.14 Data are also lacking regarding the disease’s impact on pregnant people or fetuses. Pregnant people may be more vulnerable to exposure because they more frequently engage with the health system. Developing an understanding of how the virus affects maternal and infant health—and ensuring that a vaccine or medications to treat COVID-19 are not harmful—is critical to safeguarding maternal and infant health amid the pandemic.
COVID-19 and pregnancy: What we do and don’t know
Pregnant people’s risk of contracting COVID-19
- Initial data suggest that pregnant people’s risk of contracting the coronavirus is the same as it is for adults who are not pregnant.15
COVID-19 risk to pregnant people
- CDC data found that pregnant women with COVID-19 were more likely than nonpregnant women of reproductive age to be hospitalized, admitted to the intensive care unit, and to receive mechanical ventilation.16 However, the data showed no difference in risk of death.17 While the CDC drew from nationwide reports of women of reproductive age with positive COVID-19 tests from January 22 to June 7, 2020, information on pregnancy status was not available for three-quarters of those women, limiting the cases available for analysis.
- Although they don’t seem to be disproportionately at risk of contracting COVID-19, pregnant people with COVID-19 do face health risks. There have been cases of maternal mortality and severe maternal morbidity among pregnant women with COVID-19, including cardiopulmonary complications.18
- In one study, conducted at a New York hospital observing 211 asymptomatic pregnant women, each of the women tested positive despite exhibiting no symptoms.19 This is a limited study, but it could suggest the prevalence of asymptomatic pregnant women throughout the country.
COVID-19 risk to fetuses and infants
- A small number of infants have tested positive for COVID-19 shortly after birth, but it is unclear if they were infected before, during, or after birth.20
- There have been a small number of cases of preterm birth, fetal distress, stillbirth, and infant mortality with COVID-19 infection later in pregnancy.21
- In one case of a woman with COVID-19 who had a miscarriage in the second trimester, the placenta tested positive for COVID-19.22 Another small study found evidence of injury to the placentas of pregnant women with COVID-19.23
- Data suggest COVID-19 is unlikely to be transferred via breast milk, but this is not definitively known.24
Other considerations
- A study of 108 pregnant women with COVID-19 found that 91 percent delivered by cesarean section (C-section). For comparison, the average C-section rate in the United States is 32 percent. C-sections pose greater health risks than vaginal delivery.25
- There are concerns about interruption of maternal health care as a result of strained health care capacity and reduction of in-person services due to COVID-19.26
- There are also concerns about increased risk of exposure to COVID-19 for pregnant people, as they have more exposure to health care settings.
- Women of color may experience increased risk of both maternal mortality and morbidity, and COVID-19 infection and death. In particular, the alarmingly high rates of COVID-19 among Black people compound an existing maternal health crisis. CDC data have found that Hispanic and non-Hispanic Black women were disproportionately likely to be affected by COVID-19 during pregnancy.27 Even before this pandemic, Black women were three to four times more likely to die from pregnancy-related complications and at increased risk of severe maternal morbidity.28
Women are, currently and historically, underrepresented in medical research
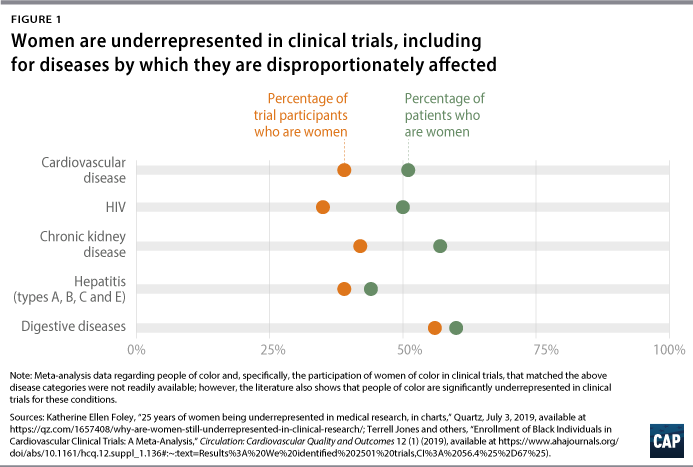
In a cross-sectional study of 43,135 published articles and clinical trial records from 1966 to 2018, researchers found that women were underrepresented in studies covering 7 out of 11 disease categories.29 Particularly stark disparities were found in research on HIV/AIDS, chronic kidney diseases, and cardiovascular diseases, as well as neoplasms (which could be a cancer characteristic), digestive diseases, neurological disorders, and hepatitis.30 The only category in which women were overrepresented was musculoskeletal disorders.31
For many of these disease categories, not only were women underrepresented in research compared with men, but the proportion of women studied was also much lower than the overall proportion of women who suffer from these conditions.32 For example, 57 percent of chronic kidney disease patients are women, yet women made up only 44 percent and 42 percent of participants in kidney disease studies and clinical trials, respectively.33 It is important to note that the study, like many studies, evaluated the inclusion of patients in a male-female binary; sex-disaggregated data often exclude nonbinary and gender-nonconforming people as well as exclude or obscure data for transgender people, who may not be categorized according to their gender identity if they are included at all. The male bias in clinical studies extended to research on animals as well: In a study of biomedical research on mammals, male bias was present in 8 out of 10 biological fields.34
People of color are also often excluded or underrepresented in clinical research, leaving women of color doubly excluded.35 An investigation by ProPublica found that African Americans, as well as Native Americans or Alaska Natives, were underrepresented in clinical trials for new cancer drugs, including for some cancers that disproportionately affect those groups.36 Other studies have found inadequate enrollment of underrepresented racial and ethnic groups in clinical trials for respiratory illness, cancer, cardiovascular diseases, and diabetes, despite greater incidence and mortality for those diseases among these groups.37
The history and justification of women’s exclusion from clinical research
U.S. policy has evolved from excluding women from clinical research to encouraging their inclusion, but women, especially women of color, remain underrepresented. In 1977, Food and Drug Administration (FDA) guidance prohibited most women of “childbearing potential” from participating in clinical trials.38 While the reasoning for this ban stemmed from protecting vulnerable populations, the exclusion led to serious health consequences for women from certain drugs.39 In 1983, the U.S. Department of Health and Human Services (HHS) established a task force on women’s health that contributed to a growing understanding that sex-based differences are significant to biomedical research.40 In 1993, the 1977 guidance excluding women of reproductive age was rescinded,41 and Congress passed the National Institutes of Health (NIH) Revitalization Act, which required the inclusion of women and “racial and ethnic minorities” in NIH-funded clinical trials and established guidelines for outreach and inclusion.42
Currently, the NIH is tasked with ensuring “the trial is designed and carried out in a manner sufficient to provide for a valid analysis of whether the variables being studied” will affect women and people of color differently than others in the trial.43 However, this standard was only applied to preclinical trials in 2014 and to animal studies in 2016.44 This requirement also applies only to publicly funded research; the majority of clinical trials in the United States are funded by the private pharmaceutical industry.45 Although women and people of color are included in clinical studies at higher rates since the 1993 NIH Revitalization Act passed, they are still underrepresented.46
Clinical trials, dominated by industry, perpetuate inequity and lack of inclusion of women and people of color
The existing structure of clinical trials can be conducive to inequity. There are different types of research, including but not limited to preclinical research, pharmacology studies, epidemiological studies, case studies, and randomized controlled trials. Clinical trials are a critical part of medical research, helping to translate basic research lab findings into new drugs and therapies.47 These trials can also provide key evidence regarding the safety and effectiveness of therapies and other interventions.
The expense associated with completing all four stages of a clinical trial—in which each phase gets progressively longer and more expensive—is high: The average price for biopharmaceutical companies to bring one new drug to market is about $1 billion.48 In addition, less than 14 percent of drugs that go through all four stages of clinical trials ultimately receive FDA approval.49 These factors make it much less likely for a drug developer to repeat an already completed clinical trial in order to increase the diversity of study participants, meaning that if women are underrepresented in drug development, it can be years before there is another opportunity to include them in future trials. In addition, study participant recruitment poses a particular challenge: Recruiting patients is one of the most time-consuming aspects of trials, eating up 30 percent of the overall clinical timeline.50 Furthermore, given historically low participation of women and communities of color in clinical trials, researchers may hold a perception that women and patients of color are more difficult to recruit or less likely to adhere to treatment regimens.51 Subsequently, private industry might not engage in such recruitment without an incentive to do so.52
Industry-sponsored clinical trials are also becoming more common among all clinical trials. Traditionally, the NIH has provided significant funding for clinical trials, and its influence—largely driven by an interest in funding trials for treatments that promote public health—provides an important counterbalance to industry-sponsored clinical trials that are more likely to be driven by profit. Indeed, NIH-funded trials are required to actively recruit women and people of color among study participants, but there is inconsistency in reporting on the inclusion of these populations, as discussed more below.53 However, between 2005 and 2015, the number of NIH-funded clinical trials shrank by more than 40 percent in part due to budget shortfalls.54 This drop has been in contrast to a rise in the number of industry-sponsored trials55—a concerning development given the commercial interests at play in industry trials as well as these companies’ reluctance to explore treatments focused on lifestyle interventions or drug comparisons that are important for public health but are less profitable.
Even with an increase in private clinical trials, the federal government can and does influence these trials. Importantly, many industry-sponsored clinical trials are based on prior trials funded by the NIH, the results of which have been released to the public domain.56 Additionally, the federal government frequently enters into public-private partnerships providing direct funding to private companies, which must also adhere to the NIH’s mandate to ensure women and people of color are included in NIH-funded clinical research. Notably, at the time of publication of this report, the White House has named five companies as the most likely candidates to produce a coronavirus vaccine, and three of these companies have received $2.2 billion in federal funding to advance vaccine development.57 Even more, the FDA also must approve therapies before they are available to the public, and the agency has issued guidance that women should be included in efficacy studies and data assessments.58 But the FDA does not currently require drug developers to recruit diverse groups of study participants to ensure that drugs have an adequate representation of women in trials before granting FDA approval. Even beyond the NIH and FDA, a host of federal agencies invest in such public health work: For example, the U.S. Department of Defense has invested in research; the CDC has conducted epidemiological and/or clinical research; the Health Resources and Services Administration has offered influential guidance on therapies; and the Agency for Healthcare and Research Quality has supported large databases that researchers rely upon.59
Studies on the lack of women in research identify justifications for their exclusion. The reasoning includes two conflicting assumptions: that women will have the same physiological responses as men and that the bodies of women and people with the capacity for pregnancy are too different and variable because of their hormones and reproductive systems.60 Other reasoning includes concerns about the impact of hormonal cycles on experimental results and population homogeneity; a desire to protect women from harmful side effects; concerns around the interaction of therapies with hormonal contraceptives; and greater costs of research on women.61 In addition, a desire to reproduce similar study conditions in research over time can create a feedback loop in which women are excluded from new studies because they were excluded in previous studies.62
However, these variable factors are precisely why the inclusion of people across gender is critical to understanding whether treatments will be effective for everyone and if they have harmful side effects. As a result of their exclusion from research and knowledge growth around the human body, medical research and practice has historically considered women, as well as others who do not fit the mold of the cisgender male body—including transgender men, nonbinary and gender-nonconforming people, and intersex people—to deviate from the norm of biological responses. This male-centric view ignores the reality that there are established sex-based differences in the incidence of and response to different diseases and health conditions, as well as responses to certain medications and therapies.
In particular, pregnant people have historically been, and continue to be, excluded from clinical trials. The primary justification for excluding pregnant people is concern over risks of experimental therapies to the pregnant person and the fetus. Other reasons include fear of legal liability; concern that pregnant people’s physiology is too complex; concern that pregnant people will not want to participate; and ambiguous and restrictive regulations around the inclusion of pregnant people, including classifying them as a “vulnerable” population.63 However, excluding pregnant people from clinical research could leave them vulnerable to adverse yet poorly understood health outcomes. Such was the case with the tragic problems created by thalidomide: This sedative medication was first prescribed in the 1950s and marketed as safe for everyone including pregnant women, but it ultimately led to birth defects in babies, including deformed limbs and nerve and blood disorders.64 Pregnancy has repeatedly been shown to affect how the body metabolizes drugs.65 Excluding pregnant people from clinical trials does not eliminate risk; it simply shifts that risk to appearing after drugs and vaccines are on the market, outside of a controlled trial setting.66
Currently, medications for conditions specific to pregnant and lactating people must be shown to be safe for these groups, but there is a dearth of research on these conditions. Additionally, medications that might be approved for the general population frequently are not required to be proven safe and effective for pregnant and lactating people.67 The FDA does not regulate dietary supplements, including prenatal vitamins, as strictly, and these products’ quality, safety, and effectiveness might not be clear until they are on the market. Notably, a recent investigation into 220 prenatal vitamin brands found that the vitamins contained various levels of lead. Even though each level was below the FDA limit, the CDC and FDA state there is no known safe level of lead, suggesting the FDA limits need to be adjusted.68 A lack of research and knowledge presents health concerns for both the pregnant person and fetus, given the increasingly common use of medication among pregnant people. An analysis of medication use in the United States between 1976 and 2008 found that prescription medication use during the first trimester of pregnancy increased more than 60 percent,69 and currently 50 percent of pregnant women use at least one prescribed medication.70
For people of color, and women of color specifically, histories of nonconsensual medical experimentation and abuse have left a legacy of distrust that may also contribute to these groups’ lack of inclusion in medical research.71 The Tuskegee study, for example, is one of the most egregious incidents of unethical and abusive medical research, in which Black men with syphilis were systematically denied treatment for decades.72 Women of color in particular have experienced violence at the hands of the medical system, from the foundation of modern gynecology built on nonconsensual experimentation on enslaved Black women, to forced sterilization campaigns targeting Native American, Black, and Latina women, to birth control pills tested on Puerto Rican women without informed consent.73 The legacies of this nonconsensual research on communities of color unfortunately continue today. For example, African Americans are disproportionately likely to be enrolled in clinical trials that do not require informed consent; a review of two decades of these “exception from informed consent” trials found that nearly one-third of patients were African American.74 In addition, while people of color are underrepresented in phase III clinical trials that test the effectiveness of new therapies, they are often overrepresented in the earliest stages of clinical trials that test drugs for safety—when there is an increased likelihood of an adverse reaction.75
People with disabilities have also experienced state-sanctioned forced sterilization and medical experimentation at the hands of medical researchers,76 and many continue to have their bodily autonomy violated in medical settings today, including in reproductive health care.77 People with disabilities continue to be excluded and underrepresented in health research, which may be influenced by distrust of medical institutions as well as a lack of accessible research design and strong informed consent protections.78
Ongoing barriers to women’s participation in clinical trials
Barriers that make it difficult to participate in clinical trials continue to exacerbate this underrepresentation in clinical research. Both women and researchers report experiencing numerous barriers to participation and recruitment, outlined below, from the logistical and financial burdens of participation to distrust or lack of understanding of the clinical process, as well as researcher bias.
Study burden
Participation in a clinical trial involves regularly scheduled site visits during which researchers collect relevant physical, cognitive, and other health information. However, attending these site visits, particularly during later trial phases that can span a few years, can pose logistical and financial challenges for study participants. Women have cited transportation—specifically, difficulties in procuring public or private transportation options; high associated costs and travel time; and logistical challenges such as inconvenient clinic locations—as one of the most difficult barriers to participating in trials, along with caregiving and child care responsibilities, concerns about taking time off, or a lack of paid leave.79
Distrust of researchers and clinical trials process
The history of nonconsensual medical experimentation has made it difficult for researchers to recruit among communities of color due to distrust and fear of further exploitation. Unfortunately, these abuses remain a concern. For example, from 2006 to 2010, dozens of incarcerated women of color in California were sterilized without consent.80 In addition, fears about the risks of participation—such as placement in the control, or placebo, group—as well as the possible negative side effects of drug exposure, including poor health outcomes or stigmatization, are barriers to women participating in trials.81
Lack of awareness
According to some studies, there is a lack of awareness about clinical trials generally as well as the clinical trial process itself, including informed consent protections. Indeed, providers serving certain racial and ethnic groups, including African American, Hispanic, and Asian patients, may not be engaged in clinical trials for various reasons—from a lack of time or availability to fears that such recruitment could interfere with the patient-provider relationship—despite the fact that referring providers are critical to broader and diverse patient recruitment.82 For people with limited English proficiency, a lack of culturally competent or translated resources to explain the study protocol, expected outcomes, and implications is also a barrier to participation. There is also a general problem with having information shared at an accessible level with all patients.83
Researcher bias and shortage
Researchers who recruit study participants can sometimes hold biases related to the recruitment of women and people of color. For example, they may perceive interactions or language barriers with such patients to be challenging or simply may not believe these patients are ideal candidates for their trial.84 Additionally, while many leading experts agree that pregnant and lactating people should be included in clinical trials, a limited number of researchers specialize in obstetrics.85
Lack of diversity among researchers
There is also a lack of diversity among researchers, particularly researchers of color, which has spillover effects on recruitment of women of color, among other underrepresented groups.86 Researchers of color are more likely to ask people of color to participate in trials; likewise, these patients are more likely to participate if asked by researchers of color.87 The lack of racial and ethnic diversity among researchers presents another barrier to women of color participating in clinical trials.
To achieve more equitable treatments, researchers and drug developers must work to build trust among women, particularly women of color, and make trials more accessible. Fears of abuse in medical research have already presented challenges in recruitment for studies of coronavirus testing and treatments.88 Building trust and providing equitable access to testing, care, and treatments are critical to ensuring that clinical studies are inclusive and rooted in informed consent. Increasing diversity among study participants and researchers will ensure the significant upfront investment in drug trials translates to treatments that are available to a broader range of patients, including women who have historically been underrepresented in clinical trials.
Inequitable research has resulted in inequitable treatments and health outcomes
It is important to consider women’s health needs in medical research because viral infections and other diseases are frequently experienced differently between sexes. Even among women, biological responses to infections and diseases may differ by race, ethnicity, pregnancy status, menopausal status, and age. The failure to collect data on women’s symptoms, coupled with low representation of women in clinical trials, can significantly undermine therapy development affecting not only women’s health but also public health more broadly. There are lessons to be learned from previous public health crises, such as Ebola and SARS, during which this problem has occurred. More importantly, there are ongoing problems—beyond COVID-19—that require additional federal investment and oversight to achieve equitable treatments for conditions that affect women the most, such as fibroids, endometriosis, and pregnancy, as well as technologies that women rely upon such as contraceptives.
Women’s health needs have been ignored in previous public health crises
Specifically, the failure to disaggregate data between men and women, and among different subsets of women, has resulted in women being misdiagnosed or diagnosed late, and conditions women experience being overlooked. For instance, data have suggested that women generally, and in particular certain subsets of women such as pregnant women, each experience Ebola, SARS, and dengue fever differently, but there have been limited data to develop a comprehensive understanding of these varied impacts. Women with dengue fever, for example, frequently experience heavy vaginal bleeding during menstruation, but the severity and frequency is not completely understood because symptoms for men and women are often not disaggregated. Furthermore, the risks for menstruating and nonmenstruating women have not been quantified.89 Similarly, pregnancy status was not systematically recorded in several countries during the SARS pandemic.90 As a result, there is not a clear understanding of SARS’ impact on pregnancies, people who are pregnant, or fetuses, though some data suggest that pregnant women with SARS have higher mortality rates, more frequently experience miscarriages, and even develop psychological conditions.91
The failure to notice pregnant people’s symptoms can also result in delayed diagnoses or misdiagnoses. According to the World Health Organization, clinical data on Ebola are limited.92 Ebola is associated with miscarriages during the first and second trimesters, but given miscarriages are common, a person presenting with such symptoms might not have been suspected as having Ebola.93 This is even more concerning during the outbreak of a viral infectious disease given pregnant people frequently interact with the health care system, which could increase their exposure to a virus. Notably, the first known outbreak of Ebola occurred in the Democratic Republic of Congo after pregnant women received vitamin injections from contaminated needles.94 Similarly, tracking only the cumulative total of infections by gender can mask who is being most affected in real time. When Ebola outbreaks have occurred, men often had higher incidence rates initially, but given women frequently work in health care and are caregivers for the sick at home, the incidence rate among women has been higher as the disease progresses.
Additionally, the failure to include women in the development of therapies or vaccines can result in adverse health outcomes for women once the drug is placed on the market. For example, among the drugs that were withdrawn from the U.S. market between 1997 and 2000, health risks were found to be greater for female patients compared with male patients for 8 out of 10 prescription drugs.95 Illustratively, after 20 years of the drug Ambien being sold to the public, it was discovered that women metabolize the drug at a slower rate than men, and the FDA recommended that the dose for women be reduced by half. Before the FDA changed its recommendation, more than 700 car accidents associated with Ambien were reported.96 Additionally, researchers have concluded, “Evidence of higher quality is needed to better understand sex differences in response to influenza vaccine,” given women report more adverse reactions to the vaccine.97
Moreover, even when trials have included women, people of color, and in turn women of color, have frequently not been included in studies and the development of therapies, which has resulted in even more adverse health outcomes for these women. For example, the vaccine against HPV, a condition that nearly every person who is sexually active will contract, has significantly advanced public health:98 According to the CDC, the vaccine has reduced “infections with HPV types that cause most HPV cancers and genital warts” by 86 percent among teens and 71 percent among young adult women.99 However, researchers have concluded that the vaccines available—Gardasil, Gardasil-9, and Cervarix—may be less effective for African American women because they do not target certain HPV strands found more frequently in African American women compared with white women.100 Perhaps consequentially, Black women are more likely to develop as well as die from cervical cancer.101
Overall, previous instances of inequitable research and funding highlight the harm that may result from not assessing the impact that diseases and their treatments can have on women. There are lessons to be learned from previous outbreaks and pandemics to improve public health in the future.
There is a lack of data on current diseases and conditions beyond COVID-19
The dearth of funding to adequately collect data and conduct research on the gendered aspects of illnesses and the different ways diseases present in women has resulted in a substantial lack of understanding around the health conditions that most affect women. This has in turn led to delays in diagnosis and ineffective treatments, leaving women and their families to suffer the consequences. Conditions that require both greater funding and additional study through clinical trials and that disproportionately affect women, and women of color especially, include the following.
Fibroids
Fibroids—noncancerous tumors that appear on the uterine wall—are a debilitating condition that, for up to half of women with the condition, manifest in heavy periods, severe pain, and reproductive issues.102 Almost 80 percent of women will have fibroids by the age of 50, making it an exceptionally common condition, but even still, there is a lack of funding for clinical trials.103 In rare cases, fibroids are associated with an increased risk of miscarriage or infertility, although most women with fibroids can successfully carry a pregnancy to term.104 For unknown reasons, women of African and Caribbean descent are two to three times more likely to develop the condition.105 There is no single treatment for fibroids. Instead, women with fibroids must manage their condition with solutions ranging from over-the-counter medications to manage their pain, to the surgical removal of fibroids, to hysterectomies. While there is an increasing amount of evidence about how fibroids develop, the number of well-conducted trials that include short- and long-term treatment options, as well as treatments for women with fibroids who wish to become pregnant despite the complications posed by their condition, is severely lacking.106
Lupus
Lupus is an autoimmune condition that can result in fatigue, joint pain, and rashes. It is a common condition, affecting 1.5 million people in the United States, 90 percent of whom are women.107 Lupus is also two to three times more likely to affect Black women.108 However, there is no known treatment for the condition, in large part due to the heterogeneity of the disease and common underlying comorbidities, such as cardiovascular disease, that makes it difficult to treat or recruit for.109 Moreover, studies have found a vast underrepresentation of Black and Latinx patients in clinical trials110—a critical oversight given the disproportionate impact of the condition on communities of color.
Endometriosis
Endometriosis is a condition caused by the growth of tissue outside the uterus, including on the ovaries, bowels, or bladder, that results in pain and menstrual irregularities. It is also widespread, affecting about 10 percent of women of reproductive age.111 Studies have found that Black women are less likely to be diagnosed with endometriosis compared with white women112 and are more likely to be misdiagnosed as having pelvic inflammatory disease—a complication arising from sexually transmitted infections.113 Regarding clinical trials of endometriosis, there have reportedly been issues around the transparency of these trials: According to a study, endometriosis clinical trials had a publication rate that was much lower compared with nonendometriosis trials published on ClinicalTrials.gov—31.4 percent in 2009 compared with 66.3 percent for completed nonendometriosis trials registered on ClinicalTrials.gov.114 Many trials were reported as inactive or halted, or the results were simply not published.
Contraception
Research and clinical trials for contraception have been sparse.115 This is a critical oversight, since almost all sexually active women of reproductive age have at some point in their lives used a form of contraception.116 While a number of available contraceptive methods exist, women need a wider range of birth control options to enhance bodily autonomy and decisions around pregnancy prevention as well as management of certain conditions, such as menstrual pain, endometriosis, and polycystic ovarian syndrome.117 Researchers are currently developing a slow-release birth control method that people would only need to take once a month, based on the way the tablet breaks down in the stomach—a possible game changer for people for whom consistent adherence to daily pills is challenging.118 The FDA has also proposed guidance that would expand the enrollment criteria and study elements for birth control clinical trials, including eliminating restrictions based on body mass index and participant age—although there are concerns about the comparison groups the FDA is using to issue this guidance.119 In sum, additional research and clinical trials for contraceptives are needed to continue expanding the array of options available in order to develop the next class of novel and innovative contraceptive options that meet the needs of all people.
Maternal health
There is a dearth of research data regarding conditions that affect pregnant and lactating people, as well as related gaps in understanding the impacts of certain medications on women. For instance, the NIH’s Task Force on Research Specific to Pregnant and Lactating Women found that in the past decade, no pharmacological study has been published on pregnant women and asthma medications.120 Similarly, research gaps exist on how autoimmune diseases; infectious diseases such as Zika and hepatitis B; chronic pain; and mental health conditions beyond depression, among others, affect pregnant people. There are also gaps on the impact of related treatments. Funding for conditions specific to pregnant and lactating people, including for low milk supply and therapies related to preterm birth, are frequently sparse. Overall, the task force experts concluded that dosing and other pharmacology information “specific to pregnancy and lactation is frequently unavailable or inadequate, despite the many physiologic changes women experience during this period, pointing to the need to enroll pregnant and lactating women in clinical studies.”121 All this is against the backdrop of a maternal health crisis in the United States and its accompanying stark racial disparities, where Black women are three to four times more likely to die from pregnancy-related complications compared with non-Hispanic white women.122 As researchers attempt to fill the aforementioned gaps, it is critical that any new treatments and therapies that are developed for maternal health center women of color—and Black women in particular.
Other conditions
Along with these conditions, women are also more affected by well-researched and funded diseases, such as heart-related conditions. Heart disease is the leading cause of death for both men and women in the United States; despite this, the condition has been studied largely based on men’s risk factors and symptoms—both of which differ significantly from women’s.123 As a result, both patients and physicians may not identify women’s symptoms as those of heart disease, and women are more likely than men to die when they do have a heart attack.124 Similarly, one study found that for cardiovascular conditions—of which women make up 51 percent of cases—women only represented 39 percent of trial study participants. Likewise with hepatitis, women make up 44 percent of all patients and yet represent only 39 percent of participants in clinical trials.125
Despite significant funding for clinical trials in these disease areas, women’s representation in these trials has been found to be comparatively lower, and this lack of inclusion has led to numerous instances of adverse outcomes from drug exposure.126
The lack of sufficient funding for treatment and other medical therapies for conditions such as fibroids, lupus, and endometriosis; the dearth of research data for important aspects of reproductive health care, such as contraception and maternal health; and the poor representation of women in more well-funded trials such as heart disease and hepatitis clinical trials represent startling oversights by the clinical research community. The conditions that women experience, whether gender specific or those that disproportionately affect women, must be addressed and treatment options developed so that women and their families are not left to suffer from a lack of access to clinical interventions that would otherwise improve their health and quality of life.
Policy recommendations need to center women when developing therapies for COVID-19 and other conditions
Inequitable research has persisted for decades, and the COVID-19 pandemic has only intensified the urgency to ensure any vaccines and therapies developed consider women’s health needs. Congress recognized nearly 30 years ago that women and people of color were not adequately represented in research and clinical trials through the 1993 NIH Revitalization Act. Now, in response to the COVID-19 pandemic, the Coronavirus Aid, Relief, and Economic Security (CARES) Act requires the HHS to regularly report to Congress data disaggregated by race, ethnicity, age, sex, geographic region, and other relevant factors for people tested and diagnosed with COVID-19.127
Despite these policies, gaps remain in the scientific and medical community’s understanding regarding how the coronavirus, lupus, HPV, endometriosis, fibroids, and other illnesses affect or differ among women, particularly women of color and pregnant and lactating women. Lawmakers, agencies, researchers, and journal publications must take additional action to assess how conditions and diseases affect women, men, and nonbinary people differently, and subsequently, they should make a concerted effort to improve these populations’ representation in clinical trials. This will not only help mitigate the COVID-19 pandemic, but will also advance research needed to address other public health problems. Overall, a more complete understanding of women’s bodies will help advance research on issues that disproportionately affect women.
Data must be disaggregated across multiple factors for prevalence and research findings
Congress should require that researchers not only collect disaggregated data on disease prevalence, but also disaggregate demographic data. Even more, once a therapy is available on the market, data should continue to be collected and assessed regarding adverse reactions and other responses. Overall, it is important for disaggregated data to be tracked over time—and not just cumulatively, given the populations most affected by a disease may change as an illness progresses.128
In particular, these data should be disaggregated based on race, ethnicity, sex, gender identity, pregnancy status, age, sexual orientation, socioeconomic status, and geographic location. They should also account for intersecting identities. For instance, it is not enough to have data disaggregated based on race and gender without also accounting for how the disease or therapy affects women of color. Additionally, it is important to consistently collect data based on gender and sex assigned at birth, recognizing that the sex that people are assigned at birth is not always consistent with their gender identity. This is critically important to ensure information is accurately collected on how diseases or therapies affect transgender and nonbinary people. The ability to assess data across multiple studies is also impeded if some data inquire about a person’s sex assigned at birth while other data reflect a person’s self-identified gender identity. Moreover, people should be able to report on their sexual orientation as well as their gender identity in order to understand how LGBTQ people are affected.
Clinical trials should be required to assess therapies’ impacts on women
Clinical researchers should be required—and held accountable—to ensure an adequate number of women are represented in clinical trials. Lawmakers should build upon the NIH Revitalization Act to ensure that women and people of color are included in all relevant NIH-funded research across every stage of research. Even more, there should be minimum standards imposed to ensure women of color, nonbinary people, and transgender people are adequately represented. Therapy developers should have to proactively justify not including women in research. To improve compliance with NIH standards among labs receiving federal funding, as well as private labs not subject to the NIH Revitalization Act, the FDA should set minimum standards for sex representation before a drug or therapy can receive the agency’s approval.129 Relatedly, the FDA should require, where relevant, clinical data to demonstrate the impact that medications and vaccines can have on pregnant and lactating people, and this information should inform dosing and safety guidelines.
It is critical to ensure pregnant people’s health needs are considered in the treatment and vaccine development for the coronavirus and other illnesses, while maintaining rigorous informed consent standards and heightened awareness of risk to both the pregnant person and fetus. Experts have repeatedly recognized that the default standard should include pregnant people in research, including clinical trials. Several expert bodies convened by government agencies, including the Task Force on Research Specific to Pregnant Women and Lactating Women, have released recommendations on the inclusion of pregnant women in clinical trials in order to develop guidance for ethical and risk considerations.130
Lastly, it is important to continue to monitor the impact that therapies have on people once a product is on the market. For instance, quality measures are frequently not disaggregated according to sex, but these measures, particularly those that track outcomes, could serve as an important mechanism to collect information once a product has gone to market.131 Health plan data and registries that the government or private industry has created to collect information from individuals about conditions or therapies can be important sources of information.
Special considerations for pregnant and lactating people
The 21st Century Cures Act required the HHS secretary to convene the Task Force on Research Specific to Pregnant Women and Lactating Women. The law required the task force to provide advice and guidance to the HHS on research gaps related to the effectiveness of therapies for pregnant and lactating women. The task force developed 15 recommendations to improve upon these gaps and delivered a report to the HHS and Congress in 2018; the task force has been renewed for an additional two years to provide guidance to the HHS on certain recommendations.132 Below, the authors of this report have summarized selected recommendations from the task force where additional improvements could advance the equitable inclusion of pregnant and lactating women if not otherwise covered in this report.
- Remove regulatory burdens: Federal policy for the protection of human subjects, known as the Common Rule, requires researchers to obtain consent from a pregnant woman and the father of a fetus when research is expected to benefit only the fetus and not the pregnant woman.133 The rule holds a narrow exception for obtaining the father’s consent under circumstances of “unavailability, incompetence, or temporary incapacity or the pregnancy resulted from rape or incest.” This rule violates the pregnant woman’s bodily autonomy and prevents the woman from solely making a decision to participate in a preclinical or clinical trial. Congress and/or the administration should amend the rule to require consent only from the pregnant woman. This change would be consistent with the remainder of the rule, which requires consent from the pregnant woman only where research is expected to benefit the woman or the woman and the fetus. Additionally, the task force recommended that the Common Rule remove pregnant women from the list of vulnerable populations, which disincentivizes researchers from including these women, and the FDA should similarly amend its policies. Several leading scientific and medical organizations, including the National Academy of Medicine, formerly known as the Institute of Medicine, in its research from as far back as 1994, recommended that pregnant women not be considered vulnerable but instead, scientifically complex.134 This designation recognizes both that pregnant women should be included in research and that those running the trials should adhere to special ethical and research design considerations when including them.135 Lastly, the task force recommended amending the risk standard required for including pregnant women in clinical trials to shift to an assumption that pregnant women should be included, unless there are health, safety, or ethical reasons for their exclusion.
- Invest additional resources and funding into research: Congress should also provide funding to expand and improve research on the safety and efficacy of therapies for pregnant and lactating people. The task force recommended improving investments into conditions and related therapies that specifically affect pregnant and lactating women, as well as research into other conditions that could affect pregnant and lactating women. Similarly, Congress should make an investment into researching how therapies already on the market affect pregnant and lactating women.
- Expand the workforce specializing in obstetrics and lactation: There also needs to be an investment made into expanding and diversifying the researchers who have expertise in obstetric and lactation pharmacology and therapeutics. The task force concluded that there is “a significant need for training programs that provide instruction in obstetric and lactation pharmacokinetics, pharmacodynamics, pharmacogenomics, and pharmacoepidemiology.”136
In 2019, the task force was extended for two years to provide guidance based on certain recommendations.137 Given the COVID-19 pandemic, this task force, or a similar body, should continue to operate to provide direction on implementing the remaining recommendations and developing new recommendations that are specific to the inclusion of pregnant people in coronavirus research.
Congress should incentivize research into conditions disproportionately affecting women and pregnant and lactating people
The federal government, unlike private industry, has a responsibility to advance research into health care conditions and medications for the benefit of the public’s health, not for profit. Even though research gaps remain, the federal government previously or currently has several ongoing clinical trials and research initiatives into the conditions that affect women the most. In fact, much of the research that has been published on pregnant and lactating women has come from federal agencies.138 Congress should allocate additional funds to relevant government agencies for the specific purpose of advancing research into health conditions where gaps have been identified. The focus of this investment should be conditions that disproportionately affect women of color as well as the unique impact that conditions and therapies have on pregnant and lactating people.
Lawmakers should exercise oversight and enforcement authority to guarantee equitable research
There must be accountability to ensure researchers actually have adequate representation in their studies. In 2015, the Government Accountability Office (GAO) published a report on the inclusion of women in clinical trials and found that the NIH generally does not record whether a trial plans to analyze outcomes based on sex or whether the trial actually does analyze such data; subsequently, this information was not reported to Congress.139 Additionally, the NIH was found to report aggregated enrollment data on participation in trials, but the agency does not report to Congress or have data readily available to the public on participation within each trial. This can mask levels of representation in trials for specific areas. The GAO reported then that “without assurance that its clinical trials are being designed and conducted as directed under the law and its implementing policy, NIH’s insight regarding the interpretation, validation, and generalizability of findings resulting from the research it supports—as these findings apply to both women and men—is diminished, potentially limiting the value of NIH-funded research.”
To improve accountability, Congress should exercise its oversight and enforcement authority over relevant federal agencies, ensuring that government-funded research as well as therapies seeking government approval adequately consider different populations’ health needs. Consistent with the GAO’s 2015 recommendations, Congress should require the NIH to disclose within its existing reports to Congress the representation of women in clinical trials, and it should report demographic data for specific diseases and areas. Additionally, the NIH should conduct, and subsequently report about, ongoing efforts to improve diverse representation in research, including recruitment efforts and practices related to gathering informed consent. Similarly, it should report to Congress specific data on COVID-19 clinical trials, and Congress should request a GAO report on COVID-19 data and clinical trials within one year.
NIH policy also encourages that agency-funded research be published in peer-reviewed journals, which can be another oversight mechanism. The NIH has stated that it considers peer reviews when awarding funding grants,140 but in particular, the agency should require peer reviewers to prioritize the representation of women, nonbinary people, and people of color.
Government alone cannot develop equitable treatments
The research community must place an emphasis on recruiting women, nonbinary people, people of color, and people with disabilities to participate in clinical trials. In 2018, the NIH launched the All of Us research initiative, which aims to enroll more than 1 million people from different backgrounds—namely race, ethnicity, age group, region, gender identity, sexual orientation, socioeconomic status, education, and disability and health status—into NIH research. The collection of information is not specific to certain diseases, with the goal of “building a diverse database that can inform thousands of studies on a variety of conditions.”141 Private industry should set a similar goal. Additionally, the goal cannot be achieved for this initiative, or for specific disease clinical trials such as those related to COVID-19, without intentionally targeting underrepresented communities. The following are steps that nongovernment stakeholders, including researchers and journal editors, can take to encourage participation from a diverse group of patients and advance more equitable treatments:
- Truly informed consent must be obtained. For any study, researchers should explain to potential research participants the purpose and importance of research. This explanation should be provided at an appropriate reading level and should be accessible for people with limited English proficiency. To this end, a 2019 journal article authored by researchers, academics, providers, and drug developers recommends providing patients with an information booklet that is jargon free, easy to understand, and includes a list of FAQs. It also advocates offering to include a friend or family member in the decision-making process if this is desired by the patient.142
- Trusted providers can help. Providers can also be important partners in recruitment, given patients are more likely to participate in a clinical trial that their provider recommends. Thus, it is important to ensure that both researchers are similarly engaging in outreach to these providers, including safety-net providers whom people of color disproportionately rely upon, and providers have adequate information on trials to inform patients.
- Clinical trials should be patient centered. Overall, researchers should aim to create patient-centered clinical trials with input from past and potentially future patients. Researchers should also adopt and strictly adhere to, as well as inform patients on, nondiscrimination and confidentiality policies and practices.143
- Financial incentives should be provided for participation. Additionally, researchers should offset logistical hurdles, including providing transportation, flexible hours, and mobile technology support.144
- Staff should reflect the community. It is also important that all staff involved in research—including recruiters, investigators, clinical coordinators, and others—are a diverse representation of the community that they are seeking to study, and all staff should be trained on cultural competency and implicit and explicit bias.145
- Journals should require adequate representation. Given peer-reviewed journals are also a key, respected mechanism for sharing the results of clinical trials, journal editors can advance equitable drug development by establishing publishing criteria that require an adequate representation of women in clinical trial studies.146 In 2018, the Center for Research on Women and Gender published an assessment that found that peer-reviewed studies frequently did not comply with the NIH Revitalization Act. The authors concluded 72 percent of the studies reviewed “did not include sex in their analyses or provide explanations as to why not,” and there was an “overall underperformance in adherence to NIH guidelines in the analysis and reporting of women and individuals from racial and ethnic minority groups.”147
Conclusion
Government agencies, private industries, and universities are undertaking an unprecedented effort to develop a vaccine for COVID-19. Although this is a critical public health step to stop the spread of the coronavirus, it remains important to ensure that any vaccine or treatment is beneficial for all. If women’s health needs are not adequately considered, therapies could prove ineffective, or even harmful, for women—and antithetical to the goal of stopping the spread of the virus.
Historically, the health needs of women, particularly women of color, and pregnant and lactating people have not been adequately considered. The history of considering men’s bodies as the norm has led to significant research gaps in how women experience various conditions and illnesses, as well as adverse and even deadly reactions to therapies that aim to prevent or treat those conditions. Moreover, health conditions that disproportionately affect women, especially Black women, have not been prioritized for research. Researchers, policymakers, and others must address these long-standing public health problems, even as they tackle the most immediate global pandemic created by COVID-19. Targeting this unequal treatment cannot be left for another day, particularly given these same Black, Native American, and Latinx communities are also being ravaged by COVID-19. A failure to address these problems simultaneously will just compound one public health crisis on top of another.
In order to confront these new and long-standing problems, Congress, federal agencies, and researchers must swiftly enact specific policies to remove remaining regulatory barriers, incentivize relevant research, and engage in thoughtful and stringent oversight and enforcement—both for research that the federal government is funding and for research that it deems safe and effective through its approvals and recommendations. Private industry, academia, and peer-reviewed journals also have a responsibility to do their part in advancing equitable therapies by recruiting and by ensuring their policies and practices foster trust among women; transgender, nonbinary, and gender-nonconforming people; people of color; and people with disabilities. Overall, relevant stakeholders must contend with the sexist and racist policies that have led to women, especially women of color, being left out.
About the authors
Jamille Fields Allsbrook is the director of women’s health and rights with the Women’s Initiative at the Center for American Progress.
Osub Ahmed is a senior policy analyst for women’s health and rights with the Women’s Initiative at the Center.
Nora Ellmann is a research associate for women’s health and rights with the Women’s Initiative at the Center.
To find the latest CAP resources on the coronavirus, visit our coronavirus resource page.